Main navigation
Cord Blood Stem Cell Storage Process With Advanced Technology
Cordlife uses FDA approved[1] automated, functionally closed and sterile AXP®II system for processing the cord blood.
Isolation of stem cells is a critical step in cord blood banking. It affects the number of stem cells that can be harvested or recovered from the cord blood. Cell recovery rates are critical because a higher number and better quality of stem cells could enhance the success of the transplant or treatment. Selection of Cord Blood Units for transplantation is linked with not only high Total Nucleated Cells (TNCs) but also relevant parameters like Mono-Nuclear Cells (MNCs) and CD34+ cells. MNCs & CD34+ have been proven to be better predictor in terms of evaluating time taken for engraftment than TNCs.[2-5]
Cordlife has adopted AXP®II System, which is a safe, sterile and automated cord blood processing technology – that ensures higher recovery of viable CD34+ stem cells (97.3%)[6] as well as of MNCs (94.5%)[6] which are more reliable parameters for potency[2][3], thus allowing better chance of a successful transplant.
A Comparison Of The Most Commonly-Used Cord Blood Storage Processing Methods[6]
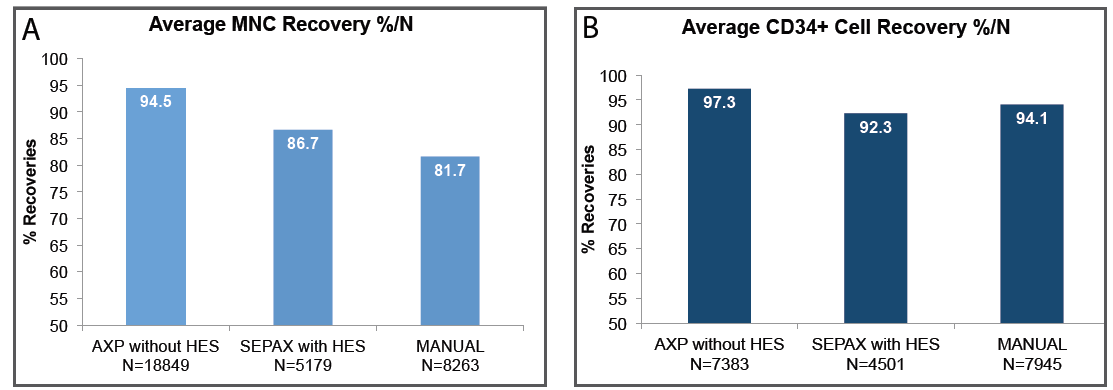
AXP®II has higher MNC and CD34+ recovery rate
The first private cord blood bank in India to have adopted an advanced processing technology that provides consistent processing of Umbilical Cord Blood without the use of Hydroxy Ethyl Starch (HES) and a high recovery rate of engraftable parameters, i.e. MNC and CD34+ cells[2-5], CD34+ recovery being higher than any other available processing systems.[7] It is also a functionally closed processing system which ensures the sterility of the cord blood by eliminating exposure to air contaminants and also cross-contamination.
AXP®II Stem Cell Technology
| Features | Benefits to You |
|---|---|
| Quality - FDA approved[1] automated cord blood processing system. - Advanced technology with new UCB protocols without using HES. | - Your baby’s cord blood storage is processed with high precision, reliable, state-of-the-art technology. |
| Automated - Controlled, smooth, functionally closed and precise separation by an advanced optical sensor. - Gives automated reading of the stem cells. | - The yield from cord blood processing is consistent, with reproducible high results. - Reduces chances of manual error. |
| Cell Recovery - Can achieve a recovery rate of as high as 96.2% Total Nucleated Cells (TNCs)[8] and 94.5% Mononuclear cells (MNCs).[6] - Viable CD34+ stem cell recovery has been found to be regularly >97%, higher than any other available processing systems.[7] | - Higher recovery of MNC and CD34+ stem cells from your baby’s cord blood are engraftable fraction after a transplant and more reliable parameters for potency[2][3], thus allowing better chance of a successful transplant. |
| Safety - Closed processing system. - Takes only 30 minutes to complete the entire processing. - Maintains maximum viability and potency of the stem cells. - Every baby's cord blood unit is fully monitored and traceable throughout each procedure. - Possesses a troubleshooting system. Even in cases of power failure, all cord blood units can be processed without error and cross-contamination. | - Storing your baby’s cord blood unit is safe and highly sterile, leaving zero opportunity for error. Prevents any sample mix-up and eliminates any possible cross-contamination of your baby’s precious cord blood unit. |
| Unique - AXP®II is calibrated and tested before every procedure. - Uniquely adapted to the qualities of each and every baby's stem cells, customizing every process accordingly. | - Prevent any sample mix-up and eliminates any possible cross-contamination of your baby’s precious cord blood unit. |
| Regulatory Approvals - FDA-cleared[1], cGMP and cGTP compliant.[7] - Complies of the highest quality standards such as AABB and FACT-NETCORD. | - Meets international health, safety and environmental requirements to ensure consumer safety. |
References
1. Scaradavou, A., et al. “Cord Blood (CB) Unit Mononuclear Cell (MNC) Dose: Effect on Transplantation Outcome and Relevance to Processing Method and CBU Selection. Blood. 2008;112:1969.
2. Patterson, J., et al. (2015). “Detecting primitive hematopoietic stem cells in total nucleated and mononuclear cell fractions from umbilical cord blood segments and units.” J Transl Med 13: 434.
3. Yoo, K. H., et al. (2007). “The impact of post-thaw colony-forming units-granulocyte/macrophage on engraftment following unrelated cord blood transplantation in pediatric recipients.” Bone Marrow Transplant 39(9): 515-521.
4. Purtill, D., et al. (2014). “Dominant unit CD34+ cell dose predicts engraftment after double-unit cord blood transplantation and is influenced by bank practice.” Blood. 124;19:2905-2912.
5. Christy Kim et al. Meta-analysis of the AXP® and SEPAX® automated cord blood processing systems. CESCA Therapeutics White Paper; 2015.
6. AXP® II System. Precision cord blood processing, stem cell harvesting, and data tracking. Thermogenesis; 2018.
7. David T. Harris. Collection, Processing, and Banking of Umbilical Cord Blood Stem Cells for Clinical Use in Transplantation and Regenerative Medicine. LabMedicine 2008 March;Volume 39;3. }
DCR No. 3423, QR 8.1-7-20, Version a



